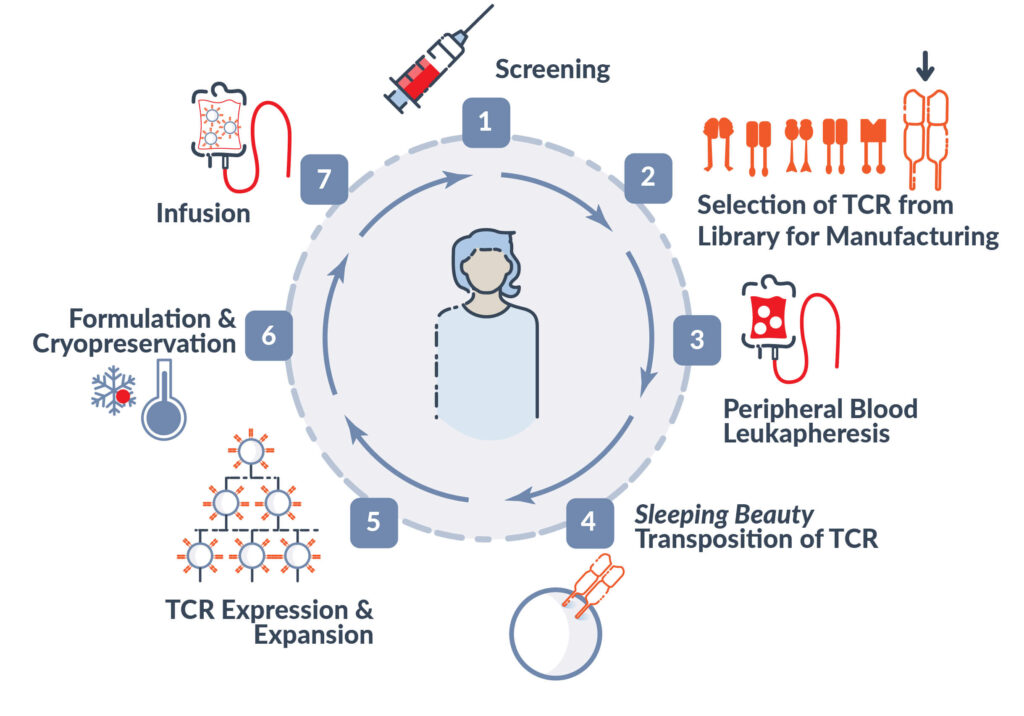
Manufacturing Process

Manufacturing Capabilities
Alaunos’ Phase 1 TCR-T cell products are manufactured in its state-of-the-art cGMP facility near the Texas Medical Center in Houston, Texas. The facility is staffed by Alaunos personnel and is fully operational for manufacturing and release of clinical product.
We are committed to improving our workflow through process and analytical development in parallel to our manufacturing team. The ability to rapidly move improvements from the lab into the cGMP suite enables our manufacturing processes to remain at the cutting-edge during the early phases of clinical development and position the program for commercial scale.