Genetic mutations are at the heart of cancer. The development and growth of tumors is dependent upon certain types of mutations, including activating driver genes (makes the cancer go) and inactivating tumor suppressor genes (stops the cancer). Targeting such mutations, therefore attacks the cancer at its root.
Solid tumors kill over 600,000 Americans every year and represent the majority of all cancers. These tumors can share mutations called “hotspots” in genes like KRAS, TP53 and EGFR (driver or tumor suppressor genes) which are critical for cancer’s survival and are therefore prevalent within both the cancer and the solid tumor population in general.
T cells are one of the body’s natural ways to eliminate cancer. The mutated gene can form a new antigen or neoantigen, which is not present on the normal, healthy cells because they do not have the mutation. Recognition of the tumor neoantigen by the T cell is coordinated by the T-cell receptor (TCR) binding through lock-and-key mechanism to the neoantigen presented on the tumor cell surface by human leukocyte antigen (HLA).
TCR-T cells specific for KRAS, p53 (from the TP53 gene), and EGFR neoantigens is an approach to adoptive cell therapy of solid tumors. We are increasing the breadth of targets by TCR-T cells through the hunTR® platform, augmenting the function of TCR-T cells by co-expression of membrane-bound interleukin-15 (mbIL-15), and generating next generation TCR-T cell therapies based on immune monitoring and correlative analyses from patients treated on the library TCR-T cell therapy trial.
hunTR® Platform
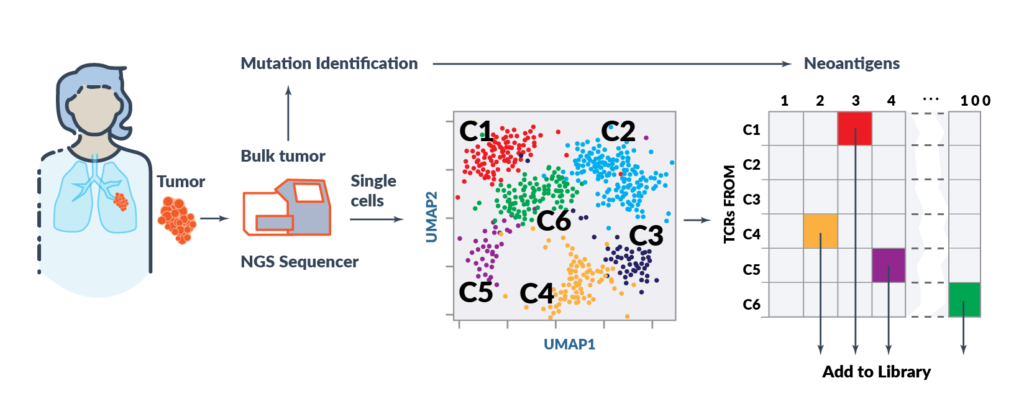
Alaunos’ hunTR® (human neoantigen T-cell Receptor) platform enables the rapid identification of new, wholly owned TCRs to further expand the Company’s growing TCR library. hunTR® is able to interrogate and deconvolute thousands of single T cells simultaneously using state-of-the-art bioinformatics and next generation sequencing. The TCRs from both CD4+ and CD8+ T cells are to maximize breadth of the TCR library. There is no need to expand TILs in this approach, so populations that do not expand ex vivo can be evaluated. This proprietary high-throughput TCR screening process permits rapid functional validation of TCRs. Based on this process Alaunos will be able to rapidly advance new TCR library candidates from the lab through to clinical translation.
mbIL-15 TCR-T cells
Potential to Promote
TCR-T Cell Persistence
Minimal Off-target Effects
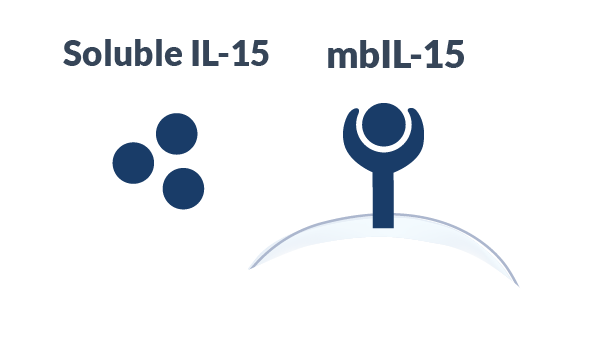
Compared Soluble IL-15
Potential Support of NK Cell Antitumor Responses in MHC-1 Deficient Tumors
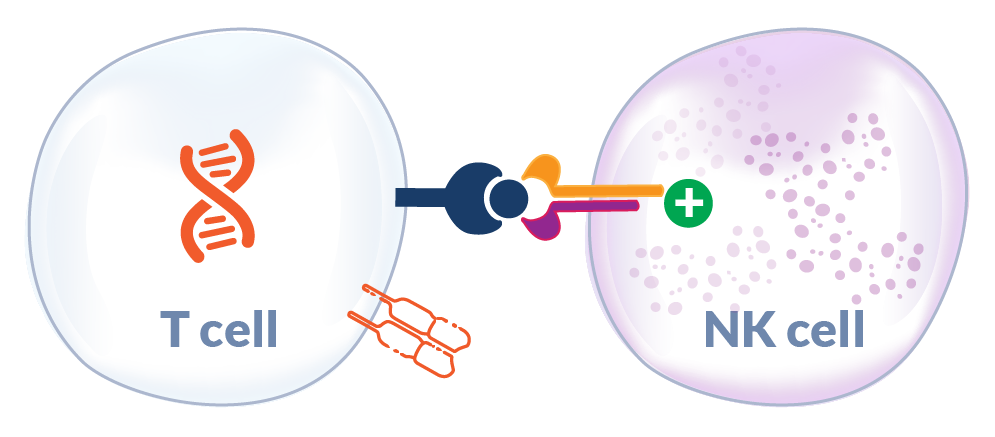
Interleukin-15 (IL-15) is a homeostatic cytokine that supports survival of T cells and other immune cells (e.g., NK cells) and is trans-presented in the context of its receptor (IL-15 Receptor-α). Elevated serum levels of IL-15 at the time of adoptive T cell therapy administration have been shown to be a key correlate in engineered T cell therapy engraftment and clinical tumor responses.
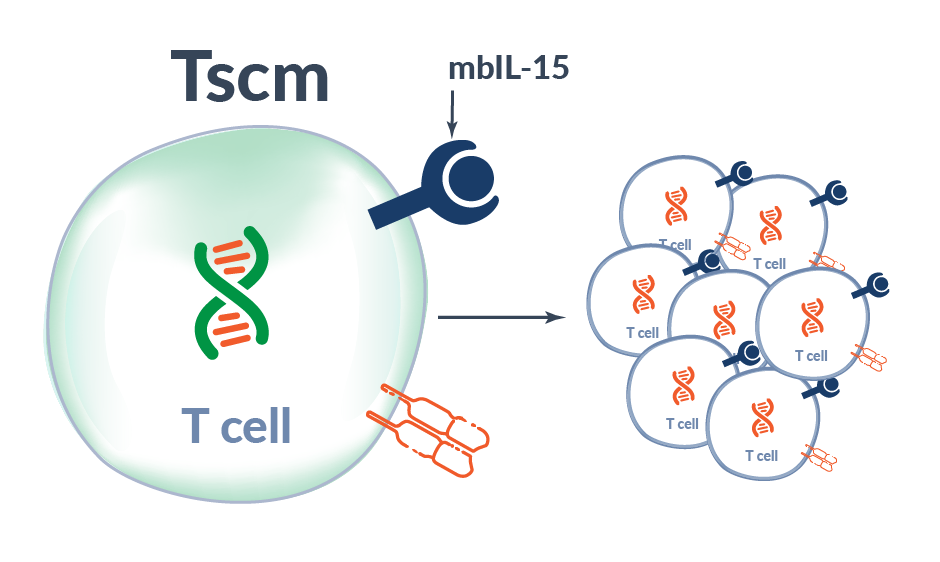
Alaunos has developed the co-expression of neoantigen-specific TCRs with a proprietary membrane-bound IL-15 (mbIL-15) to mimic IL-15 trans-presentation to increase survival and persistence of infused TCR-T cells. In pre-clinical studies, mbIL-15 promoted the development of long-lived T stem cell memory (Tscm) cells capable to survival in the absence to exogenous growth stimuli; however, upon restimulation, these Tscm cells divided to produce effector TCR-T cells with the potential to mediate tumor-killing. Moreover, by tethering our IL-15 molecule to the T cell surface, the potential for off-target toxicities observed with soluble, systemic IL-15 administration is likely reduced.
Working in synergy with our neoantigen-targeted TCR library, we believe mbIL-15 has the potential to enhance TCR-T cell therapy and deepen clinical responses targeting hotspot mutations in solid tumors.
Find out more about the science behind Alaunos’ mbIL-15 TCR-T cells here.
Sleeping Beauty Transposition
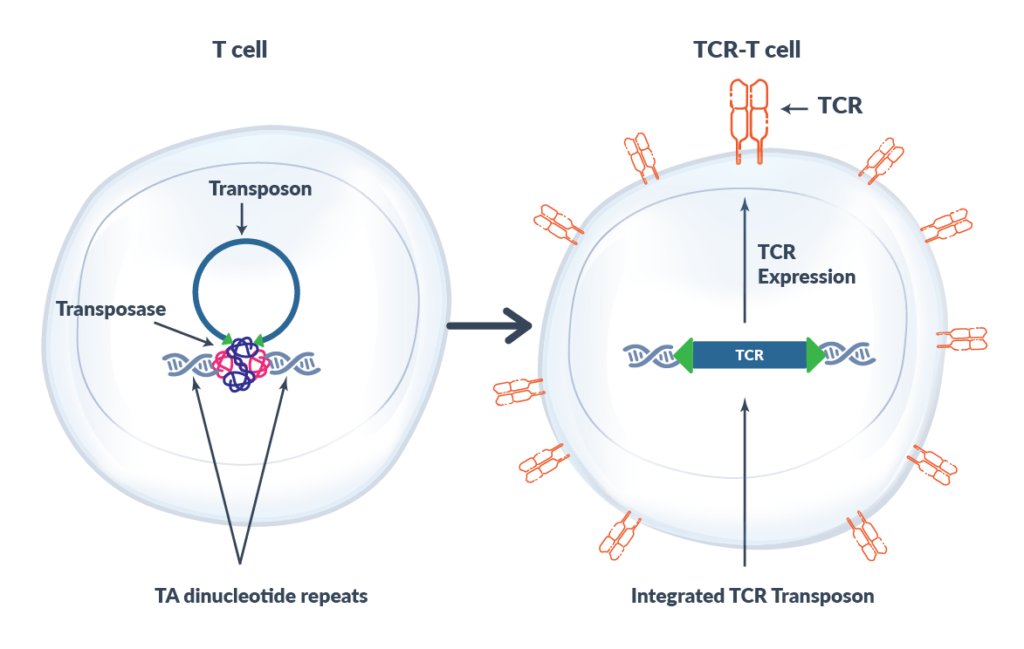
The Sleeping Beauty transposase/transposon system can be used as a non-viral gene transfer system in human cells. Sleeping Beauty transposase is briefly expressed only long enough to integrate the transposon into the genome and is then degraded and eliminated from the T cell. Sleeping Beauty transposon is inserted into TA dinucleotide repeats randomly within the human genome. Co-transfer of Sleeping Beauty transposase and transposon into the T cell results in rapid and stable expression of the introduced neoantigen-specific TCR. Sleeping Beauty’s flexibility and low manufacturing time and cost compared to other gene transfer technologies makes it an attractive tool.
To date, Alaunos’ Sleeping Beauty platform has been used to develop transposed CAR-T cell therapies, which have been used to treat leukemia and lymphoma patients with a favorable safety profile and objective clinical regressions of established disease. Alaunos has transitioned this clinical Sleeping Beauty transposon/transposase system to express TCRs and generate TCR-T cells.